Irrigation and fertigation system
Recent Techniques in Fertigation of Horticultural Crops in Israel
Abstract
Israel is a small country with a total land area of 21,000 km2, from which 20% is arable land. More than half of Israel has an arid to semi-arid climate. Approximately half of the cultivated area (200,000 hectares) has to be irrigated due to lack of rainfall and other water resources. Approximately 80% of the irrigated land in Israel uses the fertigation method, combining the application of water and fertilizers through the drip irrigation system.
The Israeli production of vegetables, flowers, ornamental plants and spices in greenhouses has been experiencing accelerated growth in recent years, reaching 3,000 hectares today. Most of the greenhouses are computerized, allowing automatic control of water, fertilizers and climate systems.
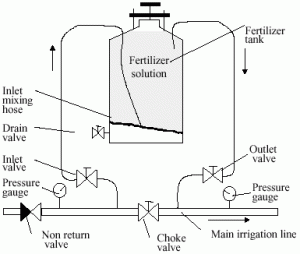
The direct delivery of fertilizers through drip irrigation demands the use of soluble fertilizers and pumping and injection systems for introducing the fertilizers directly into the irrigation system. Many Israeli companies specialize in manufacturing fertigation systems and in producing fertilizers and mixtures for their application through the drip irrigation system.
Fertigation allows an accurate and uniform application of nutrients to the wetted area, where the active roots are concentrated. Therefore, it is possible to adequate the nutrients quantity and concentration to their demand through the growing season of the crop. Consequently, recommendations have been developed for the most suitable fertilizer formulation (including the basic nutrients NPK and microelements) according to the type of soil, physiological stage, climate and other factors. Special attention should be given to the pH and NO3/NH4 ratio, nutrient mobility in soil and salinity conditions.
Planning the irrigation system and nutrient supply to the crops according to their physiological stage of development, and consideration of the soil and climate characteristics, result in high yields and high quality crops with minimum pollution.
Introduction
Israel is a small country with a total area of 21.000 km2, from which 20% is arable land. More than half of the area of Israel has an arid to semi-arid climate Near half of the cultivable area (200.000 hectares) must be irrigated due to the lack of rain and other water resources. Approximately 80% of the irrigated area in Israel uses the method of "fertigation", that combines the application of irrigation water with fertilizers. This practice contributes to the achievement of higher yields and better quality by increasing remarkably the efficiency of the fertilizer application. Greenhouse crops in Israel are fertilized exclusively through the irrigation system. The Israeli production of vegetables, ornamental flowers, plants and spices under greenhouses has experienced an accelerated growth in the last years, with more than 3.000 hectares of greenhouses nowadays Most of these greenhouses are computerized, allowing the automatic control of the irrigation, the fertilization and the climate. Hydroponics in Israel reaches a total area of 700 Has, being the main crops tomatoes, cucumbers, strawberries and flowers (roses, crisantemum, gerbera and gypsophylla). The most common growing medium is tuff (volcanic stone) which is a reactive substrate with high power of adsorption and high indigenous phosphorus content. Inert substrates as rockwool and vermiculite are also used. At the moment most of the greenhouses have an open system. The aim is to change them by closed systems in which the farmer must collect the leached solution and reuse it thus avoiding contamination
Israel is an unequaled example of the use of fertilizers by fertigation. In 1996, the Israeli farmer used an average of 115 kg N/Ha, 46 kg P2O5/Ha and 57.5 kg K2O/Ha. Over 50% of the N and P2O5, and 65% of the K2O is applied by fertigation (Tarchitzky and Magen, 1997).
Advantages of fertigation
The fertigation allows to apply the nutrients exactly and uniformly only to the wetted root volume, where the active roots are concentrated. This remarkably increases the efficiency in the application of the fertilizer, which allows reducing the amount of applied fertilizer. This not only reduces the production costs but also lessens the potential of groundwater pollution caused by the fertilizer leaching. Fertigation allows to adapt the amount and concentration of the applied nutrients in order to meet the actual nutritional requirement of the crop throughout the growing season. In order to make a correct planning of the nutrients supply to the crop according to its physiological stage, we must know the optimal daily nutrient consumption rate during the growing cycle that results in maximum yield and production quality These functions are specific for each crop and climate, and were determined in different experiments for the main crops in Israel like tomatoes, cucumbers, melons maize, etc. (Table 1). The optimal curve of consumption of nutrients defines the minimal application rate of a certain nutrient that is required to maintain a constant nutrient concentration in the soil solution. These data constitute the base of the recommendations given by the Israeli Soil Extension Service for the farmers regarding the fertigation regime for the different crops.
Other advantages of the fertigation are: (1) the saving of energy and labor, (2) the flexibility of the moment of the application (nutrients can be applied to the soil when crop or soil conditions would otherwise prohibit entry into the field with conventional equipment), (3) convenient use of compound and ready-mix nutrient solutions containing also small concentrations of micronutrients which are otherwise very difficult to apply accurately to the soil, and (4) the supply of nutrients can be more carefully regulated and monitored. When fertigation is applied through the drip irrigation system, crop foliage can be kept dry thus avoiding leaf burn and delaying the development of plant pathogens.
Drip and microirrigation have a characteristic not shared by other irrigation methods: fertigation is not optional, but is actually necessary. Fertigation provides the only good way to apply fertilizers physically to the crop root zone. On high value drip irrigated crops, such as lettuce, tomatoes, and peppers, the level of fertigation management for achieving high yields and crop qualities exceeds to what is found with other irrigation methods and crops.
* emergence ** planting
Chemical and biological guidelines for a sound fertigation
Effective fertigation requires an understanding of plant growth behavior including nutrient requirements and rooting patterns, soil chemistry such as solubility and mobility of the nutrients, fertilizers chemistry (mixing compatibility, precipitation, clogging and corrosion) and water quality factors including pH, salt and sodium hazards, and toxic ions.
Fertilizers solubility
An essential pre-requisite for the solid fertilizer use in fertigation is its complete dissolution in the irrigation water. Examples of highly soluble fertilizers appropriate for their use in fertigation are: ammonium nitrate, potassium chloride, potassium nitrate, urea, ammonium monophosphate and potassium monophosphate.
The solubility of fertilizers depends on the temperature. The fertilizer solutions stored during the summer form precipitates when the temperatures decrease in the autumn, due to the diminution of the solubility with low temperatures. Therefore it is recommended to dilute the solutions stored at the end of the summer. Fertilizer solutions of smaller degree specially formulated by the manufacturers are used during the winter.
| Table 2: Fertilizers solubility and temperatures (g/100 g water) (Wolf et al., 1985). | |||||
| Temperature | KCl | K2SO4 | KNO3 | NH4NO3 | Urea |
| 10°C | 31 | 9 | 21 | 158 | 84 |
| 20°C | 34 | 11 | 31 | 195 | 105 |
| 30°C | 37 | 13 | 46 | 242 | 133 |
Interaction between the fertilizers and irrigation water
Water quality: Many water sources in Israel have high contents of calcium, magnesium and bicarbonates (hard waters), the reaction of the water is alkaline with pH values between 7.2 and 8.5. The interaction of these waters with fertilizers can cause diverse problems, such as formation of precipitates in the fertilization tank and clogging of the drippers and filters. In waters with high calcium content andbicarbonates, use of sulphate fertilizers causes the precipitation of CaSO4 obtruding drippers and filters. The use of urea induces the precipitation of CaCO3 because the urea increases pH.
The main problem concerns phosphorus application: the presence of high concentrations of calcium and magnesium and high pH values lead to the precipitation of calcium and magnesium phosphates. Recycled waters are particularly susceptible to precipitation due to its high bicarbonate and organic matter content. The resultant precipitates are deposited on pipe walls and in orifices of drippers and can completely plug the irrigation system. At the same time, P supply to the roots is impaired. When choosing P fertilizers for fertigation with high calcium and magnesium concentrations, acid P fertilizers (phosphoric acid or monoammonium phosphate) are recommended.
Clogging: This is specially critical for drip systems that must be kept free from suspended solids and microorganisms that plug the small orifices in the emitters. In the case of clogging of the drip system by bicarbonate precipitation the use of fertilizers with acid reaction partially corrects this problem. However, acid fertilizers cause corrosion of the metallic components of the irrigation system and damage the cement and asbest pipes. Therefore, the periodic injection of acid in the fertigation system is recommended in order to dissolve the precipitates and to unclog the drippers. The following acids can be used phosphoric, nitric, sulfuric and chlorhydric. In Israel, HCl is widely used due to its low cost. Acid injection through the system will also remove bacteria, algae and slime. The irrigation and injection system should be carefully washed after the injection of acid.
Fertigation under saline conditions: Crops vary widely in their tolerance to plants, reference tables are available defining individual crop sensitivity to total soluble salts and individual toxic ions (Maas and Hoffman, 1977). When brackish waters are used for irrigation, we must bear in mind that fertilizers are salts and therefore they contribute to the increase of the EC of the irrigation water. Nonetheless, calculation of the contribution of chloride from KCl to the ove