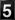Heifer care and management
Mastitis Control in Heifers and First Lactation

Table of Contents
Summary
Introduction
Use of DHI Somatic Cell Counts
Culture Milk Samples
Preventing High SCC or Clinical Mastitis at Calving
Milking Procedures to Minimize New Intramammary Infections
Antibiotic Treatment
Livestock Environment
Fly Control
Conclusions
References
Summary
Many heifers may have a mastitis infection at calving. Many of these infections are caused by the major pathogens-streptococci, Staphylococcus aureus, or coliforms. Quarters may show clinical symptoms or have elevated somatic cell counts (SCC) in milk. Some may be infected by minor pathogens that cause some irritation and elevation in milk SCC. The prevalence of infection in heifers may exceed the herd level. Heifers may become infected during the first 2 to 3 months of life, between 18 months and calving, or just prior to calving. Common causes may include damp, wet, and dirty conditions, flies, or suckling by other animals. Management factors associated with reducing risk of infection are discussed.
Introduction
In certain herds, clinical mastitis or elevated somatic cell counts can develop in first lactation cows either shortly after calving or during lactation. The pathogenic bacteria that cause the infection vary from farm to farm. Staphylococcus aureus (S. aureus) were found in 10 % of 615 heifers in 10 Washington herds. There was no relationship between prevalence of staph in the herd and infection among heifers at calving. In a Louisiana study, Nickerson collected samples from 116 pregnant and unbred Jersey heifers from four herds. Bacterial infections were present in 97% of heifers and 75% of quarters. Clinical mastitis was found in 29% of heifers and 15% of quarters. There were 2.8 infected quarters per animal, with 20-37% of the infections caused by S. aureus. Many intramammary infections in breeding age and pregnant heifers can persist for long periods of time, are associated with elevated SCC, and may impair mammary development and affect milk production after calving. Other studies have found that inflammatory responses occur in these infected quarters of pregnant heifers that subsequently reduce milk production by as much as 18%, as well as fat test. Most intramammary infections became clinical. Over 60% of staphylococcal infections continued into second lactation. There are very few spontaneous recoveries without antibiotic treatment. Consequently, S. aureus infections become potential sources of infection to the herd.
Studies conducted at USDA (Beltsville) observed that environmental bacteria could be a problem, with 7% of heifers infected by environmental streptococci and 4% with coliforms. At calving, 19% of quarters were found to be infected when heifers were housed, with 98% of the infections caused by environmental bacteria. In pastured heifers, only 8% were found to be infected, but 75% of these infections were caused by environmental organisms. A Tennessee study has suggested that heifers may pick up infections from dry cows, especially under confinement conditions.
Infections caused by the major pathogens (Streptococcus agalactiae, S. aureus, environmental streptococci, coliforms) often cause increased somatic cell counts, although we have found 30% of S. aureus infections in cows with SCC below 300,000. But, environmental staphylococci infections were quite frequent. Environmental staph usually do not cause much of an increase in milk SCC and are generally considered minor pathogens. Many environmental staph infections in heifers at calving recover by 1-2 weeks into lactation without any treatment. Although these infections could affect mammary development, many are confined to the streak canal and are eliminated during early lactation.
The above studies have examined whole herd situations rather than focusing on cows with high SCC or clinical mastitis. In heifers that develop problems around calving, the infection may have occurred at the time of calving or the preceding 1-2 weeks, or perhaps as early as the first 2-3 months of life, if calves received milk, mastitis milk, or milk replacer while grouped in pens or had an opportunity to nurse other calves.
Use of DHI Somatic Cell Counts
The DHI somatic cell count (SCC) program is useful for determining when subclinical mastitis starts to develop in first lactation. A DHI SCC score of 5 (284,000 to 565,000 actual SCC) usually indicates that an animal has an udder infection. Consult the DHI-202 herd summary sheet and the stage of lactation profile. First lactation cows should have an average SCC score of 2.0 or less during any stage of the lactation (Table 1), but especially during the first 1-40 days. Higher average scores indicate that subclinical mastitis is excessive. Cows whose scores or counts continue at 5 or 284,000-565,000 may become chronically infected and may never recover regardless of antibiotic therapy. A goal would be to have 82% or more of first lactation cows to have scores 0 to 3 and 92% with a SCC score of 4 or less (actual SCC less than 142,000) or no more than 8% scoring 5 or above.
| Table 1. Desired somatic cell counts in first lactation | |||||
|
Stage of lactation (days)
|
|||||
| 1-40 | 41-100 | 101-199 | 200-305 | 306+ | |
| Ave SCC score | 2.1 | 1.8 | 2.1 | 2.0 | 2.4 |
| Wt. Actual SCC | 124 | 108 | 126 | 124 | 146 |
Consult the individual cow SCC (DHI 210) and determine if SCC is high at first test after calving or whether SCC increases as the lactation progresses. First lactation cows should have lower SCC scores than older cows. Lowest SCC scores generally will be found early in lactation. High SCC after calving may be caused by poor environmental conditions at or before calving, ineffective dry cow therapy in treated animals, or even flies. Increases in SCC as the lactation progresses could be due to improper milking practices, faulty milking equipment, stray electricity, or poor environmental conditions.
In the example below, Heifer 1 had a low SCC score at calving but then increased to SCC score of 7 in the second month and remained high for the third and fourth months. As soon as the SCC increased for the second month, the elevated SCC should have been confirmed with the California Mastitis Test (CMT), which would indicate quarters affected, and milk cultured to determine type of infection if CMT was positive. Heifer 2 had a high SCC score at the first test after calving which remained high for the lactation. She should have been confirmed with CMT and, if positive, her milk should have been cultured. Heifer 3 had a low SCC score. No additional testing is necessary unless her DHI SCC rises above 4.0. All three heifers were from the same herd.
|
Heifer No.
|
Test after Calving | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|
SCC Score
|
|||||||||
| 1 | 0 | 7 | 5 | 7 | 2 | 0 | 0 | 1 | |
| 2 | 8 | 5 | 5 | 6 | 5 | 4 | 5 | 5 | 7 |
| 3 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 |
Culture Milk Samples
In order to diagnose the organisms causing the infection, collect aseptic milk samples from first lactation cows with clinical mastitis or high DHI SCC or first lactation cows fresh more than 7 days. If cultures reveal the presence of contagious bacteria (S. aureus, Strep. ag.), milking practices or milking equipment may need attention or other cows may have been the source. If the culture is positive for environmental bacteria (coliforms, environmental strep.), free stall conditions or calving area may be suspected, as well as certain improper milking practices.
Preventing High SCC or Clinical Mastitis at Calving
One of the keys is to reduce bacterial load at the teat end and reduce the incidence of new infection. Heifers should have a clean, dry environment at calving. Pastures for bred heifers should be free of ponds, swampy areas, etc. Also, precautions are needed to avoid high animal densities in a small area, such as all animals having access to only one shade tree. It is not known whether heifers would benefit if they were separated from dry cows. This may be more important during hot months where flies could make contact with lesions on teats of infected cows and potentially carry the infection to non-infected heifers. A good fly control program becomes an important component of any mastitis control program, especially for heifers.
Young Calves Through Weaning. Many mastitis pathogens are capable of living outside the udder (e.g., manure, wet bedding, mouth and muzzle, nostrils, teat skin, milkers¼ hands, flies, and waste milk). Calves allowed to nurse each other can spread mastitis infections into undeveloped mammary gland tissue, because mastitis-causing bacteria have been isolated from mouths. Baby calves should be isolated until several weeks after weaning before they are grouped. This should minimize the urge to suckle. New born calves should not receive colostrum or milk from cows with coliform mastitis. It is unclear whether milk from S. aureus-infected cows is a problem. There is no research evidence that it is, as long as calves cannot suckle others. However, high fly infestation has been related to teat lesions and S. aureus infections. There is the possibility that flies could transmit S. aureus infections from waste milk to teat ends.
Purchased Heifers. Any heifer purchased from another source should be cultured after calving and should be isolated from other milking animals until tested negative.
Precautions at Calving. Many mastitis infections occur at the time of calving or the preceding 1-2 weeks. First lactation cows have not had a chance to develop immunities to diseases found in the milking herd. A well-drained pasture is preferred as a calving area, with no access to ponds, swampy area, or drainage ditches. A clover-grass sod is desired, in contrast to fescue or muddy, "beaten-up" lots. Selenium-vitamin E supplementation to the ration or injections (4.5 mg Se/100 lb. body weight) to bred heifers at 2-3 weeks before expected calving have been shown to reduce mastitis after calving. The effect of vitamin E on clinical mastitis was more pronounced for first lactation than older cows. However, vitamin E levels of at least 1,000 IU/day during the dry period and 500 IU/d during lactation were more beneficial than National Research Council's recommended 100 IU/d (Weiss et al., 1997). Vitamin E and selenium also reduced retained placenta metritis, and cyctic ovaries. Other minerals and vitamins shown to reduce incidence of mastitis include vitamin A/beta-carotene, copper, and zinc. In addition, cow's should have access to fresh feed as soon as they leave the milking parlor.
Immunization during Dry Period and Early Lactation. Research conducted in California and Ohio showed that vaccination of cows in second lactation and later with E. coli J5 bacterin at drying off, 30 days after drying off, and within 24 hours of calving reduced the incidence and severity of clinical coliform mastitis during the first 90 days of lactation by four-fold. Bred heifers did not receive the vaccination. A Utah Extension publication has recommended that heifers can be vaccinated with J5 at the 7th and 8th months of gestation and at calving.
Prepartum Milking. Incidence of mastitis infections after calving was reduced in seven New York dairy herds when heifers were first milked 14 days before calving. Milk yield was unaffected. Two years later, four herds had continued prepartum milking of heifers because of easier adaptation to milking. The April 25, 1997, issue of Hoard's Dairyman described a New York dairy farm where 2-year-old heifers were milked a minimum of 2 weeks prior to calving, preferably 4-6 weeks (Wilson, 1997). The practice acclimated heifers to the milking environment and cut down on congestion and soreness. Heifers were put in the holding area for 1-2 days before being brought through the parlor. After several days of coming through the parlor, teats are wiped. After a couple more days, milking machines were attached. This practice may be considered for herds with mastitis problems at calving; however, heifers will secrete no colostrum at calving. Thus, new born calves will need frozen colostrum, preferably first colostrum from older cows to acquire needed immunoglobulins against disease.
Milking Procedures to Minimize New Intramammary Infections
The general recommendation has been, where possible, to milk first lactation cows before older cows who could be carrying subclinical mastitis infections. This was based on the premise that heifers would not be infected. However, many studies have found that this is not the case. The general recommendation is still valid, but other practices should be implemented to minimize infected heifers at calving. If the milking herd can be grouped, a separate group for first lactation cows is recommended, which can be milked first and fed differently. If first lactation cows cannot be separated from infected or high SCC cows, problem cows with clinical mastitis and those receiving antibiotic therapy must be milked last, or milking units must be disinfected with sanitizer after milking infected cows.
Antibiotic Treatment
Dry Cow Therapy. New infections can be found in many heifers, either at calving or in early lactation. As many as one-third of these infections may be caused by S. aureus. Often these S. aureus infections, if untreated, become clinical and recur throughout the first lactation and into the second lactation. Louisiana studies have examined the feasibility of giving antibiotic therapy to heifers (Nickerson et al., 1995). A dry cow product containing penicillin and dihydrostreptomycin was administered at the first, second, or third trimester of pregnancy in 35 bred heifers from four herds. Although prevalence of infection and SCC was reduced by treatment in all three groups of heifers, heifers dry-treated during the second trimester of pregnancy demonstrated greater reduction in mastitis and SCC at calving. Treat heifers with dry cow treatment at 60 days before expected calving date. Consult with your veterinarian for treatment options and choice of antibiotics. Properly clean and disinfect teat ends before and after treatment. Wash teats with a sanitizing solution and then dry with either a paper or cloth towel. Dip teats in an approved teat dip and blot dry after 30 seconds contact time. Scrub teats with alcohol pads before partially inserting tube into teat (one-eighth inch). Teat dip again after treatment. Turn heifers into clean and dry environment. Check milk for presence of antibiotic residue at 3 to 5 days after calving and before milk is put into milk tank.
Lactating Cow Therapy. In Tennessee, a lactating cow antibiotic treatment containing either cloxacillin or cephapirin was administered at 7 days before expected calving in heifers (Oliver et al., 1992). In a subsequent study, cephapirin was given at 14 days before calving. Treatment of pregnant heifers at 14 days prior to expected parturition with cephapirin lactation therapy gave greatest reduction in intramammary infections, with greater milk production and little risk of antibiotic residue in milk at 3 days after calving unless heifers calved shortly after treatment. Use treatment precautions indicated under Dry Cow Therapy. For herds with a high incidence of mastitis at calving, or high average SCC at first DHI test among first lactation cows, either prepartum milking or prepartum antibiotic therapy may have considerable merit. If antibiotic treatment is used, remember to follow label withholding recommendations for discarding milk. It is recommended that treated animals should be tested with an antibiotic residue test at 3-5 days after calving and milk discarded until it tests negative. In addition, there are no mastitis infusion products that have been approved for pre-calving heifers. Such a practice becomes extra-label drug use and must be practiced under the direct supervision of a veterinarian.
Livestock Environment
Control programs for heifers should be designed to reduce the number of mastitis-causing bacteria at the teat end. Sanitation in the young calf's environment, such as hutches, and pens for older heifers is important. Hutches and pens should be well bedded, clean, dry, and comfortable. Lots and pastures should be managed to prevent muddy areas where heifers would lie down. Well drained paddocks are preferred, with no access to ponds, swampy areas, or streams and ditches. Filthy, damp, or muddy pens, lots, or pastures continually expose the teat end to a barrage of bacteria. (see VCE Publication 404-252, Dairy Loafing Lot Rotational Management System, 1994.)
Fly Control
Biting flies traumatize the teat end. Flies also carry a number of mastitis-causing organisms that can colonize these teat lesions. Heifers should be isolated from infected lactating or dry cows. Elimination of fly breeding sites is one aspect of fly control. Flies breed in decaying feed or manure that has accumulated, including exercise yards, calf pens, and box stalls. Other options include backrubbers, feed additives and ear or tail tags. In one trial where tail tags contained insecticide, only 1 of 100 heifers had a mastitis infection compared to 18 of 100 untagged heifers.
Conclusions
The future of the dairy herd depends upon prevention of mastitis infections, including prevention of infections in first lactation cows. Once infected, many lactating cows do not respond to antibiotic treatment. Since most mastitis infections are caused by bacteria, control depends upon reducing the numbers of pathogens near the teat end and avoiding transfer of infection from cow to cow. For herds with a high incidence of mastitis at calving, or high average SCC at first DHI test among first lactation cows, either prepartum milking or prepartum antibiotic therapy may have considerable merit.
References
Galphin, S. P., Jr. 1997. Reducing intramammary infections in heifers utilizing tail tags. p. 145-149 in Proc. 36th Annual Meeting, National Mastitis Council
Matos, J. S., D. G. White, R. J. Harmon, and B. E. Langlois. 1991. Isolation of Staphylococcus aureus from sites other than the lactating mammary gland. J. Dairy Sci. 74:1544-1549
Nickerson, S. C., W. E. Owens, and R. L. Boddie. 1995. Mastitis in dairy heifers: Initial studies on prevalence and control. J. Dairy Sci. 78:1607
Oliver, S. P., M. J. Lewis, B. E. Gillespie, and H. H. Dowlen. 1992. Influence of prepartum antibiotic therapy on intramammary infections in primigravid heifers during early lactation. J. Dairy Sci. 75:406-414
Roberson, J.R., L.K.Fox, D.D.Hancock, J.M.Gay and T.E. Besser. 1994. Ecology of Staphylococcus aureus isolated from various sites on dairy farms. J. Dairy Sci. 77:3354-3364
Weiss, W. P., J. S. Hogan, D. A. Todhunter, and K. L. Smith. 1997. Effect of vitamin E supplementation in diets with a low concentration of selenium on mammary gland health of dairy cows. J. Dairy Sci. 80:1728-1737
Wilson, K.O. 1997. Streamlined dairy premilks its heifers prior to calving. p. 348 in April 25 issue of Hoard's Dairyman